Teva Issues Voluntary Nationwide Recall of One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure | FDA
Teva Issues Voluntary Nationwide Recall of One Lot of Anagrelide Capsules, USP 0.5 mg Due to Dissolution Test Failure

FDA announces recall of platelet-reducing medication due to risk of clotting or other adverse cardiovascular outcomes

Teva Initiates Voluntary Nationwide Recall of One Lot of Topotecan Injection 4 mg/4 mL (1 mg/mL) Due to Presence of Particulate Matter | FDA

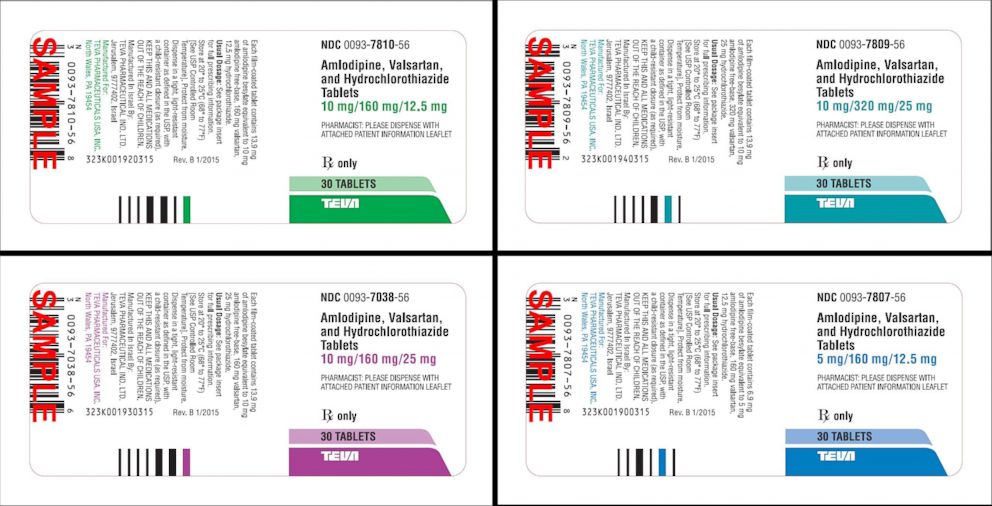
Teva Pharmaceuticals USA Issues Voluntary Nationwide Recall of all Amlodipine/Valsartan Combination Tablets and Amlodipine/Valsartan/Hydrochlorothiazide Combination Tablets that are Within Expiry | FDA



/cloudfront-us-east-1.images.arcpublishing.com/gray/UVAUY3N3VBKKPJZ24MSVDZL62I.png)









/cloudfront-us-east-2.images.arcpublishing.com/reuters/CCBS56SBRRKUDM7JEVVE3UOQEE.jpg)